Abstract
Introduction: For most acute myeloid leukemia (AML) patients (pts) an allogeneic stem cell transplantation (HSCT) offers the highest chance of cure. Recently, the European LeukemiaNet (ELN) published updated recommendations on the risk classification in AML pts. In addition to the risk at diagnosis, which is now based on cytogenetics and the mutation status of several genes, a dynamic adjustment according to the measurable residual disease (MRD) status in favorable (fav) and intermediate (int) risk pts at informative time points - such as prior to an allogeneic HSCT - was recommended. A validation of the new ELN2022 risk classification has not been reported.
Methods: We retrospectively analyzed 522 AML pts who received an allogeneic HSCT between 2000 and 2021 in first complete remission (CR, 54%), second CR (11%), CR with incomplete count recovery (CRi, 13%), or relapsed/refractory (22%) at our center. Median age at HSCT was 59 (range 16-76) years (y), 53% of pts were younger than 60 y. Conditioning regimens were myeloablative (27%), reduced-intensity (23%) or non-myeloablative (50%). At diagnosis, cytogenetics, and the mutation status of CEBPA, NPM1, and FLT3-ITD were assessed. Using next-generation sequencing we analyzed a panel of 54 recurrently mutated genes in myeloid malignancies (MiSeq platform, Illumina). In pts with material available in remission prior to HSCT, the MRD status based on NPM1mutations, BAALC, MN1, and WT1 expression was evaluated as published previously (Jentzsch 2021). MRD positivity (MRDpos) was defined as positivity of any of the analyzed markers. Median follow up after HSCT was 3.0 y.
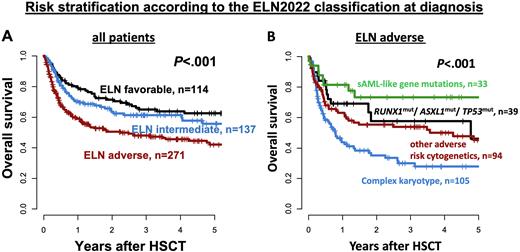
Results: ELN2022 risk at diagnosis was 22% fav, 26% int, and 52% adverse (adv). Older pts (≥ 60 y at HSCT) had significantly worse ELN2022 genetic risk (P<.001). Comparing the ELN2017 and the new ELN2022, 83% (fav), 70% (int), and 90% (adv) of pts kept their allocation, while 15% and 2% of fav ELN2017 risk pts changed to int and adv ELN2022, 30% of int ELN2017 risk pts changed to adv ELN2022 & 1% and 10% of adv ELN2017 risk pts changed to fav and int ELN2022. Analyzing the c-statistic (area under the curves [AUC]) produced by the ELN2017 and ELN2022 for relapse (AUC2017 = 0.64 vs AUC2022 = 0.64, P=.21) and overall survival (OS) prediction (AUC2017 = 0.60 vsAUC2022 = 0.57, P=.06), the ELN2022 did not perform significantly better. However, the ELN2022 risk at diagnosis impacted cumulative incidence of relapse/progression (CIR, P<.001) and OS (P<.001, Figure 1), which was especially seen in pts < 60 y (CIR P<.001, OFS P<.001), but less so in pts ≥ 60 y at HSCT (CIR P=.002, OS P=.10). At diagnosis, outcomes did not differ significantly with respect to the specific genetic abnormality defining the ELN2022 fav or the ELN2022 int risk. Also a high or low FLT3-ITD allelic ratio (0.5 cut) within the ELN2022 int risk did not result in distinct outcomes (CIR P=.13, OS P=.30). In contrast, within pts harboring adv ELN2022 risk, CIR (P=.05) and OS (P<.001) significantly differed according to the presence of specific genetic abnormalities; i.e. a complex karyotype (at 3 y: CIR 59%, OS 30%), other adverse cytogenetic aberrations (at 3 y: CIR 50%, OS 54%), adverse-risk gene mutation (i.e.ASXL1, RUNX1, or TP53, at 3 y: CIR 47%, OS 58%), or secondary AML-like gene mutations (at 3 y: CIR 30%, OS 73%). When we adjusted fav and int risk pts according to the MRD status at HSCT as proposed by the ELN2022 recommendations, 49% of fav risk pts at diagnosis had persisting MRD and were reclassified as int risk at HSCT while 53% of int risk pts at diagnosis were MRD negative and reclassified as fav risk at HSCT. Outcomes of MRD-adjusted fav risk pts improved (at 3 y: CIR 12%, OS 75%), while that of MRD-adjusted int risk pts (at 3 y: CIR 53%, OS 49%) was similar to that of adv risk pts transplanted in morphologic CR/CRi (at 3 y: CIR 47%, OS 54%).
Conclusion: In the context of an allogeneic HSCT, the proposed risk assessments by the ELN2022 significantly impacted outcomes at diagnosis as well as after adjustment according to MRD status prior to HSCT of AML pts. However, it did not perform significantly better than the previous ELN2017 risk stratification. The FLT3-ITD allelic ratio did not significantly impacted outcomes within the ELN2022 int risk, justifying the removal from the risk stratification. Noteworthy, in pts receiving allogeneic HSCT, secondary AML-like gene mutations did not associate with adverse outcomes when no other adverse-risk characteristics were present.
Disclosures
Jentzsch:Jazz Pharmaceuticals: Honoraria; Novartis: Honoraria; Pfizer: Honoraria. Metzeler:Daiichi Sankyo: Honoraria; Pfizer: Consultancy; Jazz Pharmaceuticals: Consultancy; Novartis: Consultancy; Celgene/BMS: Consultancy, Honoraria, Research Funding; Curis: Research Funding; Astellas: Honoraria; AbbVie: Honoraria. Herling:Novartis: Honoraria, Research Funding; Roche: Honoraria, Research Funding; Jazz: Honoraria, Research Funding; Janpix: Honoraria, Research Funding; Abbvie: Honoraria, Research Funding; EDO-Mundipharma: Honoraria, Research Funding; Takeda: Honoraria, Research Funding. Merz:Janssen: Honoraria; BMS Celgene: Honoraria. Vucinic:Novartis, Gilead Kite, Takeda, MSD, BMS Celgene, Abbvie, Amgen: Honoraria; MSD, BMS Celgene, Novartis, Gilead Kite, Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sobi, BMS Celgene: Other: travel, accommodations, expenses. Schwind:Novartis: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal